Research Themes
-

Microvascular Blood Flow
Our core hypothesis is that red blood cell (RBC) mechanics are a causal factor in the symptoms of microvascular diseases. In microvessels (<0.1mm diameter) the flow characteristics depend on the RBCs and their unique mechanics. These mechanics are known to be altered in diseases such as diabetes and malaria, but exactly how this affects the flow is not yet clear. The reason this is important is that the endothelial cells (ECs) that line the vessels sense forces from the flow and use them to regulate blood flow. Failure of flow regulation leads to the major symptoms of microvascular diseases, so the interaction between RBCs and ECs is of critical importance.
-

Aqueous Humour Dynamics
Increased pressure in the eye is the major risk factor and only treatable target for glaucoma. The pressure is controlled by the flow of aqueous humour (the clear fluid in the front of the eye) across tissue that is resistant to flow out of the eye. In glaucoma, the ‘outflow resistance’ increases and so does the pressure. There remain important questions on how the resistance is normally regulated and what goes wrong in glaucoma. As scientists propose answers to these questions, they require tools to evaluate whether the answers are correct – this requires technology for measuring the resistance. We use highly collaborative engineering approaches to provide improved ways to measure ‘outflow resistance’.
-

Other Collaborative Projects
We believe that collaboration and interdisciplinary work are the pillars of great science. We partner with labs across Imperial and other institutions to combine our technical expertise, in projects such as the RELAVENT ventilator made in response to the COVID-19 crisis.
Microvascular Blood Flow
Measurement of blood flow in microchannels
We use microfluidic technologies to build models of microvessels, through which we flow human blood samples. Our custom-built microparticle image velocimetry, optical densitometry and flow control systems capture unique experimental measurements of the blood flow.
By chemically modifying the blood samples, we can mimic the changes to RBCs observed in patients in a controlled way, enabling us to elucidate specific causes for changes that we observe.
Key people: Gladys Diaz-Armas, Maryam Sajjad + Simon Tupin (alumnus)
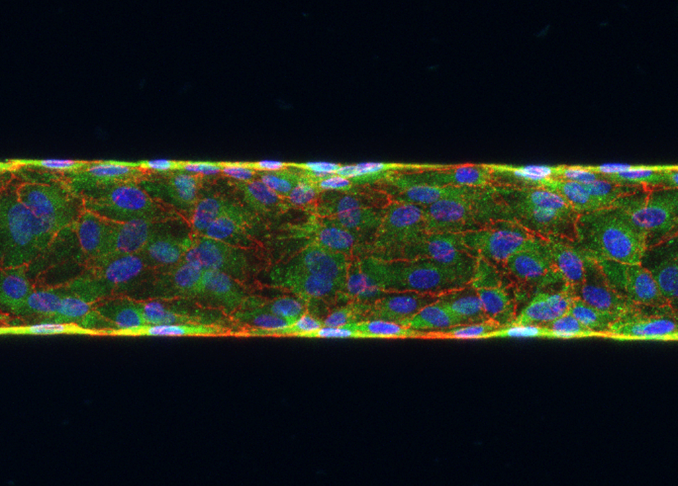
The effect of blood flow on the endothelium
We build in vitro models by vascularising microvessel-sized channels inside PDMS and hydrogels. We then perfuse these vessels with blood and compare the biological response of the ECs to those that are perfused with cell culture media under the same average mechanical forces.
We use a range of different vessel designs and modified blood samples to address the question: ‘what differences in flow meaningfully affect EC response’?
Key people: Ioana Esanu + Adele Lam & Josefin Jansson-Edqvist (alumni)
Collaborators: Anna Randi
Numerical models of microscale blood flow
Numerical models have the potential to provide important information that cannot be measured. In theory, a model can provide higher resolution in space and time, and can be used to make predictions in environments where a measurement is not possible. Our modelling approach is directly inspired by our experimental measurements and depends strongly on them for validation.
We model the red blood cells as a continuum, and where their concentration is higher, the blood is locally more viscous. This has been shown to be an efficient alternative to modelling individual red blood cells that has the potential to scale up to full microvascular networks.
Key people: Daniel Harwood
Aqueous Humour Dynamics
To measure whether a given drug or gene affects outflow resistance, scientists need to be able to measure it. The iPerfusion system was designed to provide accurate and precise measurements of this resistance. It has been optimised to work with ex vivo, postmortem and in vivo models of multiple species. There are currently 18 iPerfusion systems, each hand-built and installed in world leading labs across the world, which have been used to produce data for more than 40 publications.
Key people: Simon Tupin & Michael Madekurozwa (alumni)
Collaborators: Darryl Overby (Imperial, Bioengineering), Ross Ethier (Georgia Tech, Biomedical Engineering), Dan Stamer (Duke, Opthalmology), Simon John (Columbia, Opthalmology), Krish Kizhatil (Ohio State, Opthalmology), Fiona McDonnell (Utah, Medicine)
The iPerfusion system
Other Collaborative Projects
(more coming soon…)
During lockdown, like many teams of engineers across the world, we put together a small team to address the shortage of ventilators. In our solution, JAMVENT, we identified a way to deliver ICU-level ventilation using only on-off valves (patent pending). Compared to most ventilators, which use proportional valves, this increases robustness and reduces power usage and complexity – why Forbes called it a ‘Frugal Engineering Marvel’.
These features make the device ideal for Low and Middle Income countries where there remains a severe shortage of reliable ventilators for Malaria, TB, pneumonia etc. and our startup, rebranded as Relavent, is currently seeking funding to support this important mission.
Key people: Michael Madekurozwa (alumnus) + Jimmy Moore, Jakob Mathiszig Lee, Jennifer Frattolin, Dan Watson, Willy Bonneuil (engineering) + Liz Hughes, Steve Atkinson (startup)
Relavent
Fast Scan Cyclic Voltametry (FSCV) is a method used for real-time quantification of neurotransmitters in biological systems. Reliable calibration is essential for interpreting FSCV, but existing calibration flow cells required significant and ongoing troubleshooting with pulsing, leaking, flow inconsistencies and dead volume being major causes of common challenges. The Hashemi lab at Imperial Bioengineering asked for our help to design a reliable calibration rig using low cost materials, and this is what we came up with.
Key people: Parry Hashemi (collaborator, Imperial Bioengineering)